Philippines acquires COVID-19 test kits that yield results in at least 45 minutes | ABS-CBN
ADVERTISEMENT

Welcome, Kapamilya! We use cookies to improve your browsing experience. Continuing to use this site means you agree to our use of cookies. Tell me more!
Philippines acquires COVID-19 test kits that yield results in at least 45 minutes
Philippines acquires COVID-19 test kits that yield results in at least 45 minutes
ABS-CBN News
Published Apr 18, 2020 03:40 PM PHT
|
Updated Apr 18, 2020 10:31 PM PHT
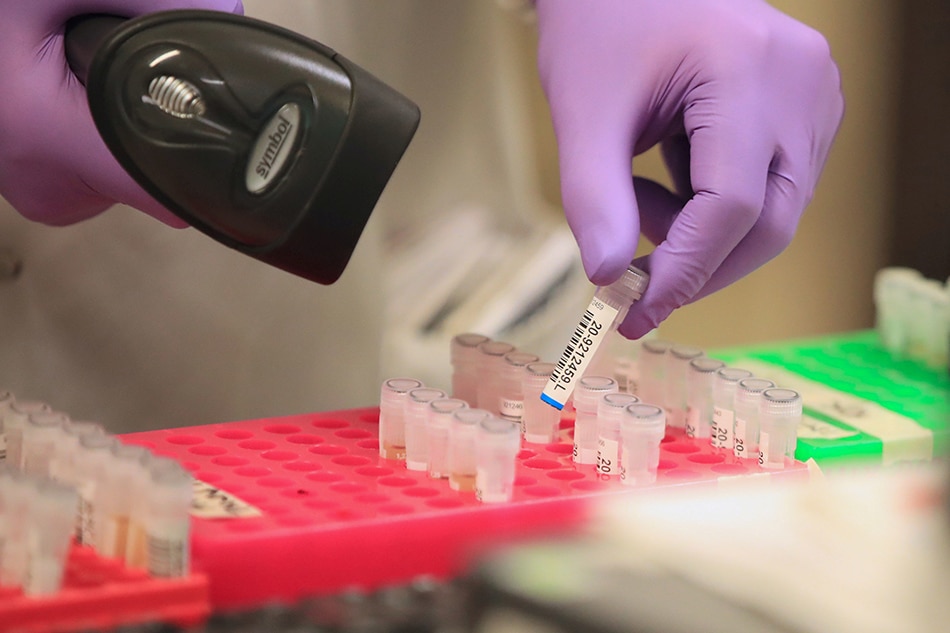
MANILA - The Philippine government has acquired new test kits that can detect coronavirus in 45 minutes, Cabinet Secretary Karlo Nograles said Saturday, as the country boosted its testing capacity.
MANILA - The Philippine government has acquired new test kits that can detect coronavirus in 45 minutes, Cabinet Secretary Karlo Nograles said Saturday, as the country boosted its testing capacity.
In a virtual press briefing, Nograles said the GeneXpert test kit, a rapid, real-time RT-PCR test intended for the qualitative detection of SARS-CoV-2 -- the virus that causes COVID-19-- will yield faster results compared to other test kits.
In a virtual press briefing, Nograles said the GeneXpert test kit, a rapid, real-time RT-PCR test intended for the qualitative detection of SARS-CoV-2 -- the virus that causes COVID-19-- will yield faster results compared to other test kits.
"Ang GeneXpert ay lab-based pa rin siya pero 45 minutes, less than an hour ang turnaround time," said Nograles, spokesperson of the Inter-Agency Task Force for the Management of Emerging Infectious Diseases (IATF-EID).
"Ang GeneXpert ay lab-based pa rin siya pero 45 minutes, less than an hour ang turnaround time," said Nograles, spokesperson of the Inter-Agency Task Force for the Management of Emerging Infectious Diseases (IATF-EID).
(GeneXpert is still lab-based but the turnaround time is 45 minutes, less than an hour.)
(GeneXpert is still lab-based but the turnaround time is 45 minutes, less than an hour.)
ADVERTISEMENT
The Philippine government earlier procured close to 3 million additional COVID-19 test kits as the country begins targeted mass testing for the disease.
The Philippine government earlier procured close to 3 million additional COVID-19 test kits as the country begins targeted mass testing for the disease.
Of this number, some 900,000 are polymerase chain reaction (PCR) test kits and 2 million are rapid test kits.
Of this number, some 900,000 are polymerase chain reaction (PCR) test kits and 2 million are rapid test kits.
PCR test kits include reagents and other chemicals used to check if a patient’s sample has the COVID-19 virus.
PCR test kits include reagents and other chemicals used to check if a patient’s sample has the COVID-19 virus.
Such kits can only be used in laboratories with PCR machines. The PCR lab technique allows the detection and amplification of genetic material in body fluids or samples. It has long been widely used in molecular biology.
Such kits can only be used in laboratories with PCR machines. The PCR lab technique allows the detection and amplification of genetic material in body fluids or samples. It has long been widely used in molecular biology.
Like other PCR test kits, it requires nose and throat swab samples from a patient.
Like other PCR test kits, it requires nose and throat swab samples from a patient.
Meanwhile, rapid test kits or immunoassay testing uses blood samples.
Meanwhile, rapid test kits or immunoassay testing uses blood samples.
Such kits are unable to detect the COVID-19 virus. Instead, it measures a patient’s antibodies through the blood sample.
Such kits are unable to detect the COVID-19 virus. Instead, it measures a patient’s antibodies through the blood sample.
Philippine health authorities have so far tested more than 42,000 individuals since the virus emerged in Wuhan City in Hubei province, China.
Philippine health authorities have so far tested more than 42,000 individuals since the virus emerged in Wuhan City in Hubei province, China.
As of Friday, COVID-19 has sickened 5,878 people, including 387 deaths and 487 recoveries.
As of Friday, COVID-19 has sickened 5,878 people, including 387 deaths and 487 recoveries.
Read More:
Karlo Nograles
IATF
test kits
GeneXpert test kit
virus
coronavirus
2019 Novel Coronavirus
2019-nCoV
2019 Novel Coronavirus acute respiratory disease
2019-nCoV ARD
ADVERTISEMENT
ADVERTISEMENT